Introduction
Naphtha, a versatile hydrocarbon mixture, plays a crucial role in the petrochemical industry. Its chemical composition, which varies depending on its source and refining process, includes alkanes, cycloalkanes, and aromatics. Recent research has focused on refining crude oil into valuable chemicals through thermal and catalytic methods, showcasing the industry's innovation in refining practices.
Companies like Coolbrook are revolutionizing industrial processes by developing technologies that aim to reduce CO2 emissions. As the industry adapts to fluctuating energy supplies and raw material availability, studies demonstrate the potential to use CO2 and hydrogen to sustainably produce olefins, alcohols, and fuels, potentially reshaping naphtha's role. Additionally, initiatives like Neste's green hydrogen project in Finland signal a shift towards more sustainable production methods that may impact naphtha's traditional uses.
With the ongoing transformation in the petrochemical sector, naphtha continues to play a crucial role.
Chemical Composition of Naphtha
Naphtha, a versatile hydrocarbon mixture, is pivotal in the petrochemical industry, with its composition ranging from C5H12 to C9H20, though often including higher molecular weights. The specific hydrocarbon blend in naphtha—comprised of alkanes, cycloalkanes, and aromatics—depends on its source and the intricacies of the refining process.
Cutting-edge research, like that led by principal scientist Ibrahim A. Abba, delves into thermal and catalytic methods to refine crude oil into a spectrum of valuable chemicals, each with distinct properties and developmental stages. Their work exemplifies the innovation in refining practices that can influence naphtha's composition and utility.
Amid this innovation, companies like Coolbrook have garnered accolades for technologies that could revolutionize industrial processes, including those involving naphtha. Coolbrook's rotating technology, recognized by The Financial Times, aims to replace fossil fuel combustion and drastically reduce CO2 emissions.
In the evolving landscape of petrochemicals, the adaptation to fluctuating energy supplies and raw material availability is crucial. Studies referenced in DOI: 10.1016/j.jcat.2023.07.012 demonstrate the potential to use CO2, a climate change contributor, in concert with hydrogen to sustainably produce olefins, alcohols, and fuels, which could reshape naphtha's role in the industry.
Furthermore, with the backdrop of Europe's declining naphtha consumption, which is projected to hit a 48-year low, initiatives like Neste's green hydrogen project in Finland represent a shift towards more sustainable production methods that may also affect naphtha's traditional uses. As the industry faces the challenges of expensive natural gas in Europe, alternative production methods and the importation of raw materials like ethylene, as indicated by the International Energy Agency, highlight the ongoing transformation in the petrochemical sector, where naphtha plays a crucial role.
Boiling Point and Fractions of Naphtha
Naphtha, a volatile flammable liquid hydrocarbon mixture, plays a critical role in the chemical industry and as a feedstock for producing a myriad of products. Comprising a complex range of hydrocarbons with a boiling point between 30°C and 200°C, naphtha is categorically segmented into light, heavy, and full-range based on the boiling point spectrum. Light naphtha is rich in paraffins and naphthenes, making it a prime choice for gasoline blending and an essential feedstock for ethylene and propylene production via steam cracking.
Heavy naphtha, with its higher aromatic content and boiling range, serves as a feedstock for catalytic reforming, which is used to produce high-octane gasoline components as well as aromatics like benzene, toluene, and xylenes. Full-range naphtha encapsulates the entire boiling range and is versatile, often employed in petrochemical operations for solvent applications or as a basal component for further refinement.
In the pursuit of more sustainable practices, the refining and utilization of naphtha are subject to environmental scrutiny. Significant reductions in emissions have been seen, such as the decrease of nitrous oxide emissions from 137 thousand tons to less than 10 thousand tons since the Emission Trading System implementation in 2005. This demonstrates the industry's ability to adapt and improve its processes in response to environmental regulations.
Technological advancements continue to emerge, as evidenced by Coolbrook's innovative rotating technology aimed at decarbonizing industrial sectors, potentially reducing CO2 emissions by 30% in heavy industries. This aligns with the industry's ongoing research into thermal and catalytic methods for converting crude oil to chemicals, offering multiple technology options for different crude types and varying capital intensities.
Amidst this evolution, the physical characteristics of crude oil, such as density and sulfur content, dictate the refining process and the naphtha produced. Lighter crude oils tend to yield more light hydrocarbons, allowing for the production of high-value products like gasoline and jet fuel through simple distillation. This intricate relationship between crude oil properties and naphtha fractions underscores the technical complexity inherent in fuel procurement and management.
Physical Properties of Naphtha
Naphtha, a clear to pale yellow fluid known for its distinctive scent, boasts a low viscosity and density that enhance its volatility. Its specific gravity typically lies between 0.65 and 0.75, coupled with a flash point that often falls below freezing, marking its high flammability. This hydrocarbon mixture is non-soluble in water, yet exhibits solubility in various organic solvents.
With innovations in renewable energy and carbon utilization gaining momentum, the chemical industry is exploring the use of carbon dioxide, a byproduct of civilization, in the sustainable production of fuels and other chemical reactants. In particular, the transformative rotating technology developed by Coolbrook, a recipient of The Financial Times' Tech Champion 2022 award, aims to revolutionize major industrial sectors by replacing fossil fuel combustion. This technology has the potential to achieve temperatures up to 1700°C and significantly reduce CO2 emissions.
Amidst advancements, it's crucial to consider the environmental impacts of chemical substances and their production processes. Carbon dioxide, for instance, is a well-documented greenhouse gas that contributes to global warming. Its comparison to other gases is measured in carbon dioxide equivalents, underscoring its role in climate change. Interestingly, as industries seek to reduce their carbon footprint, the principles of green chemistry become increasingly relevant, striving for processes that can adapt to fluctuating energy supplies and raw materials, particularly from renewable sources.
Understanding the properties and environmental implications of substances like naphtha is essential for energy and utility professionals with decades of experience, such as those with backgrounds in large-scale project development and renewable power projects. The knowledge shared by experts like Rice, with over 40 years in the energy sector, and those with a history in forestry and energy conservation, like Brian and Alyssa, is invaluable in navigating the evolving landscape of fuel procurement and energy production.
As the industry progresses, companies are encouraged to harness the expertise of seasoned professionals and leverage cutting-edge technologies to enhance sustainability and reduce environmental impact. Naphtha's role and characteristics, when considered alongside these broader initiatives, highlight the complex interplay between traditional petrochemicals and the pursuit of innovative, eco-friendly solutions.
Uses of Naphtha
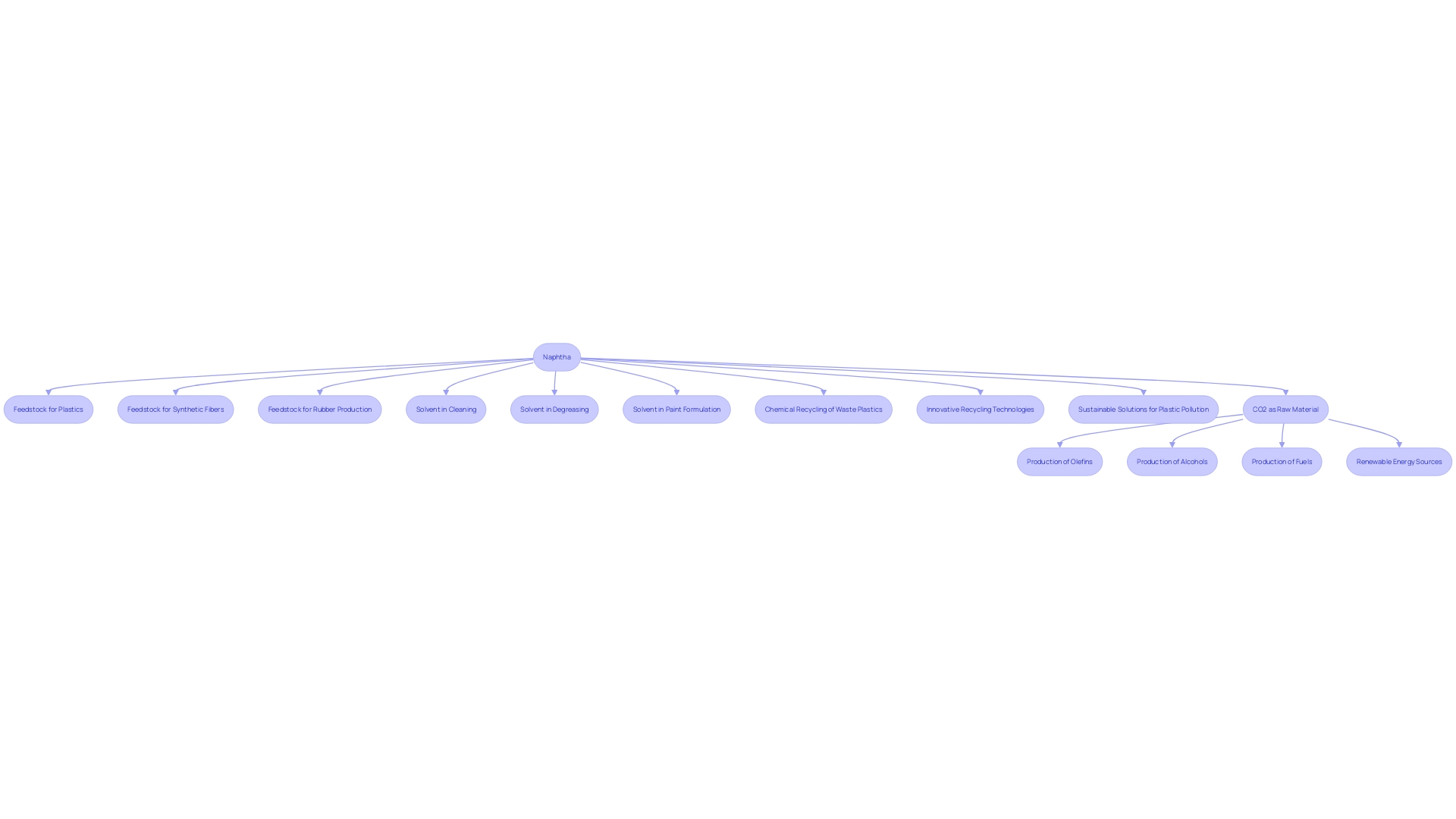
Naphtha plays a pivotal role in the petrochemical industry, serving as a critical feedstock for the creation of essential materials such as plastics, synthetic fibers, and rubber. These materials are integral to numerous applications, from reducing food waste through better packaging to ensuring the sterility of surgical instruments. However, the imperative to tackle plastic pollution has spurred chemical engineers to pursue innovative recycling technologies for less recyclable plastics and to develop new polymers that are easier to recycle.
As a solvent, naphtha is indispensable in various industrial operations, aiding in cleaning, degreasing, and the formulation of paints and coatings. Its versatility is underscored by its use as a foundational component in gasoline production, as well as its utility as a fuel in power generation and heating—a testament to the energy sector's reliance on chemical engineering solutions to meet both consumer and industrial needs efficiently and sustainably.
The ongoing drive to minimize environmental impacts while enhancing process efficiency and reducing costs is echoed in the narrative of chemical engineering. Industry experts emphasize the discipline's crucial role in addressing some of the most pressing societal and scientific challenges through the application of core principles. These principles are not only central to the field's identity but are also instrumental in shaping a more sustainable and cost-effective future.
Reflecting on the dynamic nature of the industry, current research highlights the potential of using carbon dioxide—often seen as a climate antagonist—as a valuable raw material in conjunction with hydrogen and other reactants to produce olefins, alcohols, and fuels. The success of such processes depends on their adaptability to fluctuating energy and material supplies, a challenge that the field of chemical engineering is uniquely equipped to tackle, especially as we pivot towards renewable energy sources like solar and wind power.
Production Process of Naphtha
Naphtha, a versatile hydrocarbon mixture, is primarily derived from two processes: the distillation of crude oil and the stabilization of natural gas condensate. The distillation of crude oil, which involves heating and separating the oil into various fractions, results in naphtha as a lighter fraction due to its lower boiling point. Additionally, naphtha can also be obtained through stabilizing natural gas condensate, which is a byproduct of natural gas production. This hydrocarbon blend is critical in industries due to its role as a feedstock for producing ethylene, a fundamental building block for numerous everyday products, ranging from antifreeze to textiles.
The production of naphtha and its utilization as a petrochemical feedstock are closely monitored within the Emission Trading System (ETS), ensuring environmental compliance and promoting sustainability. Researchers, like Ibrahim A. Abba and his team, are investigating innovative methods to refine crude oil into chemicals, with a focus on thermal and catalytic technologies. These efforts are part of a broader push towards using carbon dioxide in industrial processes, including the manufacture of olefins, alcohols, and fuels, which may involve naphtha as a raw material alongside sustainable reactants.
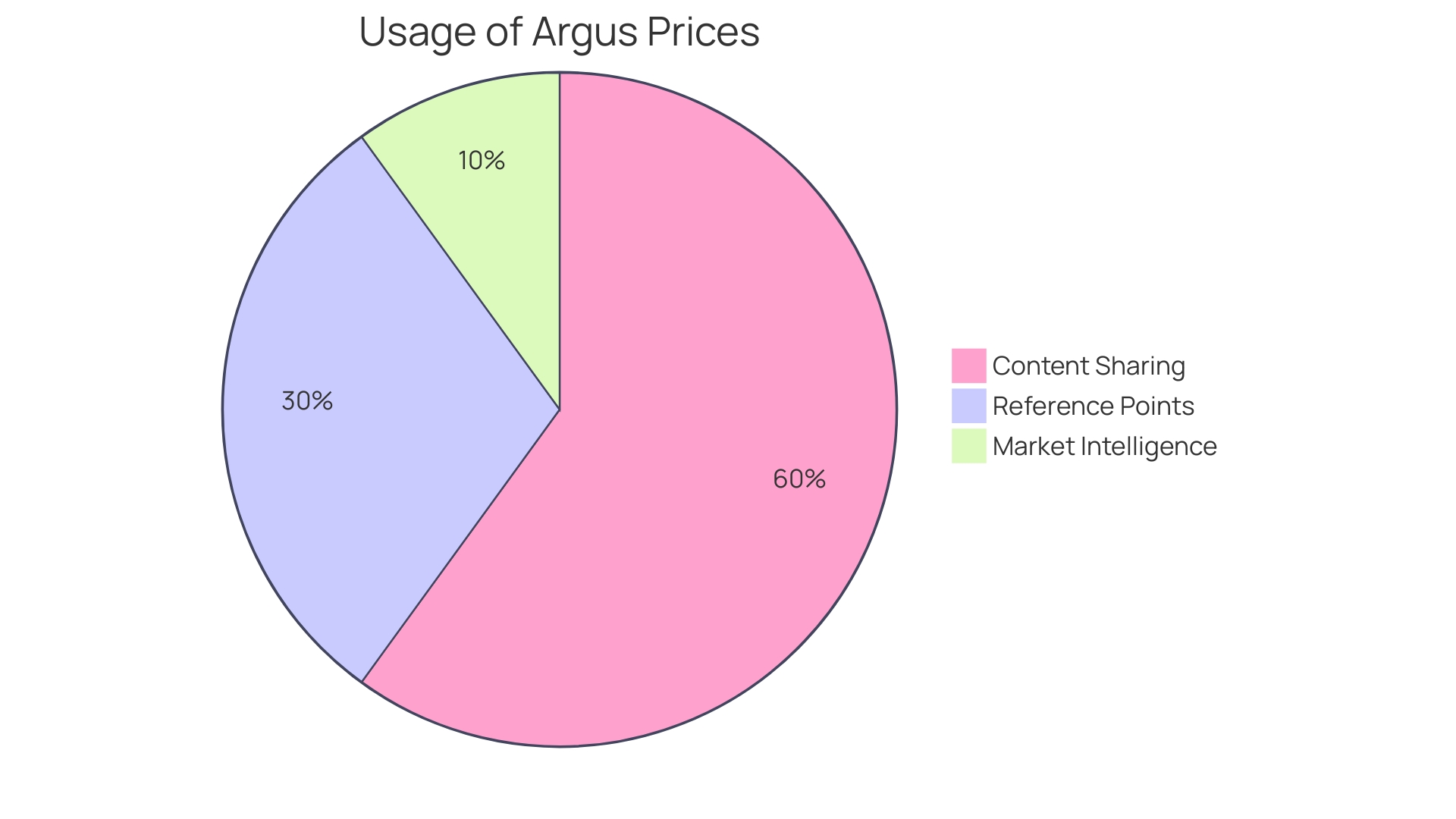
The global naphtha market is influenced by various factors, including consumption patterns, which are subject to change due to environmental concerns and market dynamics. European naphtha consumption, for instance, is anticipated to reach a 48-year low, reflecting shifting demands and the ongoing evolution of the energy and petrochemical landscape. Amidst these changes, accurate pricing and market intelligence, such as those provided by Argus, play a pivotal role in comprehending the true value of naphtha and other commodities. These insights are essential for businesses to navigate the complex market conditions, particularly as the industry seeks to balance economic growth with environmental stewardship.
Safety Considerations and Health Hazards
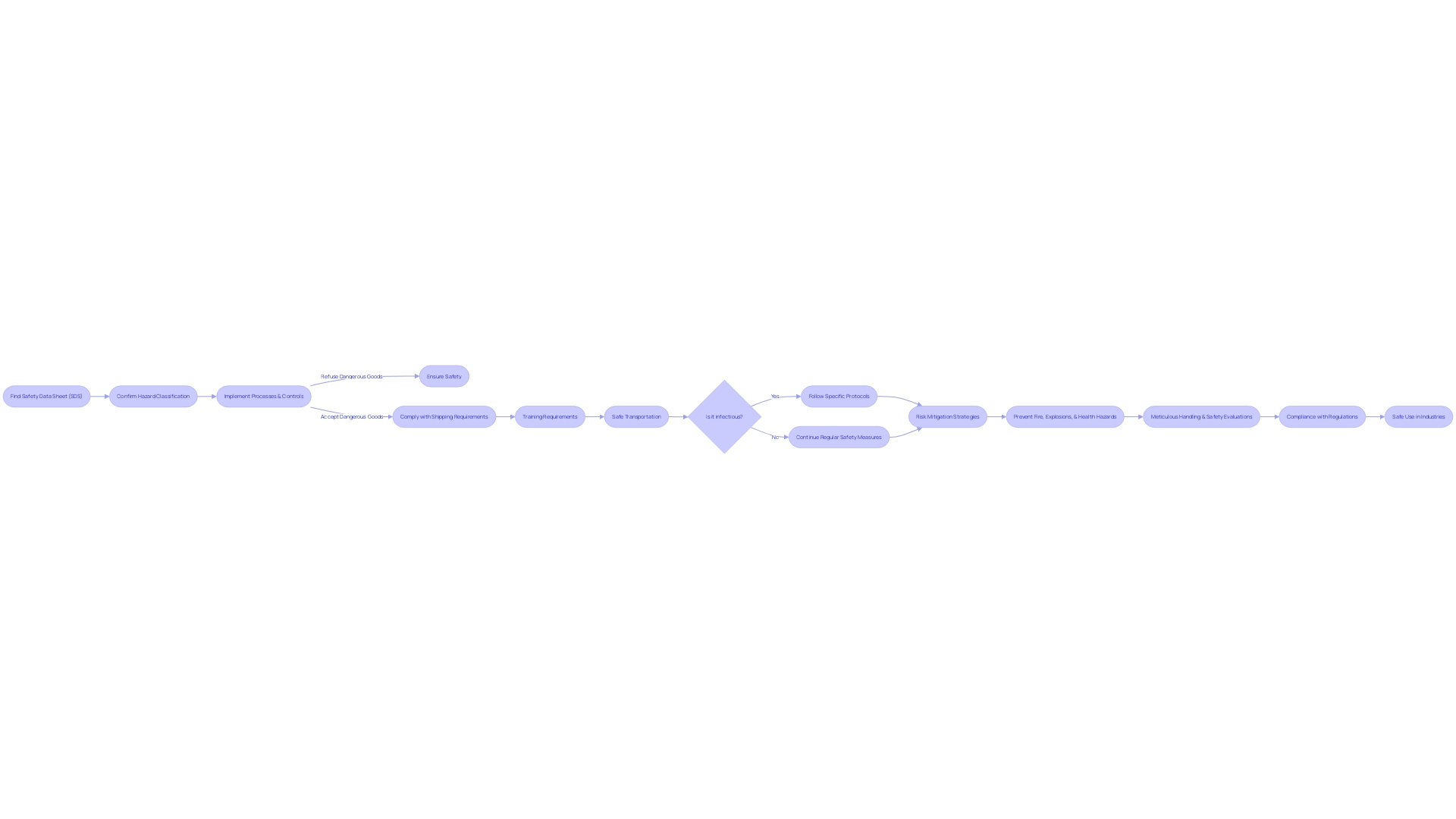
Navigating the safety challenges associated with naphtha requires vigilance and adherence to strict protocols. Naphtha's highly volatile nature means it can easily ignite from a spark or open flame. Consequently, work environments must be well-ventilated, and comprehensive safety measures must be rigorously implemented to mitigate the risks of fire or explosions. For example, Crystalgen Inc. emphasizes the importance of meticulous handling of hazardous materials, including naphtha, to safeguard lab personnel from severe health risks.
The health implications of naphtha are not to be underestimated either. Skin contact or inhaling vapors can result in irritation, while exposure to higher concentrations poses significant health hazards. Drawing parallels to the stringent safety standards applied to California's utility companies to prevent catastrophic wildfires, as explored in a case study by Michael Wara of Stanford Law School, similar meticulous safety evaluations and risk mitigation measures are essential when dealing with naphtha.
In the broader context, companies like Coolbrook have been recognized for their innovative approaches to decarbonization, showcasing the industry's potential to adapt and improve safety while also addressing environmental concerns. Additionally, Todd Newsome's extensive experience in operational regulatory compliance underscores the importance of managing hazardous products to minimize risks and maintain essential supply chains.
As the naphthalene sulfonate market expands, particularly in the Asia Pacific region, the surfactant segment is poised to drive demand due to increased construction activities. With the construction industry's shift towards sustainability and energy efficiency, the safe handling and use of chemical precursors like naphtha become even more critical.
Comparisons with Other Hydrocarbon Products
Naphtha emerges as a critical player in the portfolio of solvents and fuels, particularly due to its unique boiling point range that caters to applications necessitating swift evaporation. Unlike heavier fuel oils and diesel, naphtha transitions from liquid to gas at lower temperatures, which can be advantageous in specific processes. However, the trade-off includes its elevated volatility and a less impressive energy density when juxtaposed with gasoline and diesel. These characteristics demand careful consideration in applications where energy content is paramount. Additionally, naphtha's lower flash point makes it less ideal for aviation compared to kerosene and other specialized fuels, underscoring the importance of matching fuel properties to their intended use.
In the realm of chemical engineering and fuel technology, advancements are continually shaping our understanding and utilization of various fuels. Research spearheaded by the likes of Ibrahim A. Abba and his team of engineers and scientists has shed light on the nuances and potential of converting crude oil to valuable chemicals through thermal and catalytic methods. These efforts are not just technically intricate but also strategically diversified, providing a tapestry of options to refine crude types into different products, each with its own economic and operational considerations.
The innovative use of carbon dioxide, a notorious greenhouse gas, as a feedstock for synthesizing fuels and chemicals represents an elegant pivot towards sustainable practices in the industry. As new technologies like Coolbrook's decarbonization approach gain recognition, the potential to revolutionize the production of high-temperature industrial processes without fossil fuels becomes more tangible, aiming to significantly slash CO2 emissions.
In light of the environmental and operational challenges, the transition towards cleaner energy carriers such as e-fuels presents a promising avenue. E-fuels, synthesized from renewable energy sources, offer an array of options from e-methane to e-kerosene, with the versatility to serve as both fuel alternatives and energy storage solutions. Their role becomes increasingly significant as the transportation sector explores low carbon alternatives to traditional fuels.
The evolving landscape of fuel use and environmental stewardship is exemplified by the success stories in emissions reduction, such as the marked decrease in nitric acid emissions since the implementation of the ETS rules. This highlights the potential for informed choices and technological innovation to drive progress in reducing the carbon footprint of fuels, including naphtha, within the broader context of the industry's pursuit of efficiency and sustainability.
Conclusion
Naphtha plays a crucial role in the petrochemical industry, with ongoing research focusing on refining practices and sustainable production methods. Companies like Coolbrook are revolutionizing industrial processes to reduce CO2 emissions, while initiatives like Neste's green hydrogen project signal a shift towards more sustainable methods. Naphtha serves as a feedstock for various industries, and advancements in technology, such as Coolbrook's rotating technology, aim to decarbonize industrial sectors.
Understanding the relationship between crude oil properties and naphtha fractions is crucial for fuel procurement and management. Naphtha's physical properties and environmental implications are important considerations in the renewable fuels industry. It serves as a critical feedstock for essential materials like plastics and synthetic fibers, and its versatility extends to solvent applications and fuel usage.
The industry's focus on sustainability and reducing environmental impact is evident in ongoing research and technological advancements. The production and utilization of naphtha are closely monitored for environmental compliance and sustainability. Safety considerations and adherence to strict protocols are essential in handling naphtha.
Accurate pricing and market intelligence are crucial for businesses operating in the complex naphtha market. In conclusion, naphtha's role in the petrochemical industry remains crucial amidst ongoing transformations. The industry's focus on refining practices, reducing emissions, and exploring sustainable production methods highlights its adaptability and innovation.
Understanding the physical properties, environmental implications, and safety considerations of naphtha is essential for professionals in the renewable fuels industry. With the pursuit of efficiency and sustainability, naphtha continues to play a significant role in fuel procurement and energy production.




