Introduction
Kerosene, a widely used hydrocarbon fuel, plays a crucial role in sectors such as aviation, heating, and power generation. Understanding the molecular structure of kerosene is essential for comprehending its characteristics and performance.
In this analysis, we delve into the complex nature of the kerosene molecule, exploring its composition, properties, and implications across various applications. From advancements in synthetic kerosene production to the quest for sustainable alternatives like Flexiforming technology, we explore the technical insights and analytical perspectives of the renewable fuels industry. Join us as we unravel the intricacies of kerosene and its potential in a decarbonizing world.
Understanding the Kerosene Molecule
Kerosene, a prevalent hydrocarbon fuel, plays a pivotal role in various sectors including aviation, heating, and power generation. The mastery of its molecular structure is essential to understanding its characteristics and performance. This intricate analysis delves into the complex nature of the kerosene molecule, its composition, properties, and implications across diverse uses.
The International Conference on synthetic sustainable aviation fuel showcased advancements in synthetic kerosene production, including plans for the first commercial synthetic kerosene factory in Amsterdam. This endeavor seeks to leverage synthetic sustainable kerosene in European aviation by 2050, underscoring the potential of this fuel in a decarbonizing world. Polycyclic Aromatic Hydrocarbons (PAHs), organic molecules composed of fused benzene rings, are integral to kerosene's molecular structure.
They significantly influence chemical processes that result in the formation of soot and other carbonaceous nanoparticles. Despite PAHs constituting nearly 30% of all carbon in the universe, their formation process, particularly involving free radical reactions, is still enigmatic. Recent research has shed light on the formation of PAHs, notably naphthalene, the simplest PAH representative.
This reaction is believed to occur in the gas phase via radicals found in combustion flames and around carbon-rich stars, providing a novel understanding of our galaxy's chemistry and carbon balance. However, while kerosene satisfies specific aviation requirements like energy intensity and long range, its combustion can produce undesired byproducts, and heat transfer cooking can pose safety risks. As the energy transition becomes unavoidable, the quest for alternatives such as Sustainable Aviation Fuels (SAF) and hydrogen escalates.
In this context, technologies like Flexiforming, provided by Unifuel.tech, offer a promising solution. Flexiforming can be implemented in an idle hydrotreater or reformer, lowering capital expenditure and carbon intensity. This technology allows operators to determine their decarbonization pace, contributing to the optimization of kerosene use and the development of sustainable alternatives, thus propelling us towards a greener future.
Chemical Composition of Kerosene
The chemical makeup of kerosene, largely composed of alkanes, is characterized by singular carbon atom bonds in each kerosene molecule. The carbon atom count of these alkanes typically falls within the decanes, undecanes, and dodecanes categories, ranging from 10 to 16 carbon atoms. These alkanes are commonly referred to as kerosene molecules.
In the context of refining, it has been established that capacity expansion can be a more economical approach than setting up new refineries. However, the feasibility of such an expansion greatly depends on the size and scale of the refinery.
Smaller refineries, lacking the benefits of scale and capacity for upgrades, grapple with elevated operational costs and dwindling competitiveness, leading to their gradual phasing out. Yet, the concept of repurposing these struggling refineries into renewable fuel production facilities is under consideration.
Innovations like Flexiforming, offered by Unifuel.tech, open up additional opportunities for monetizing renewable naphtha by utilizing the kerosene molecule. The implementation of this technology in an idle hydrotreater or reformer can reduce both capital expenditure and carbon intensity by utilizing the kerosene molecule.
It allows operators to choose the pace of their decarbonization, presenting a personalized solution for flexiforming applications. In addition, thermochemical techniques have shown promise in reducing the viscosity of oil and enhancing its flow in reservoirs, by generating heat and inert gas, thus increasing the temperature of the oil's kerosene molecule in the reservoir. According to studies, it is also suggested that the molecular structure of certain hydrocarbons, specifically the kerosene molecule, significantly influences the efficiency of photochemical reactions that can produce sustainable aviation fuel. Furthermore, advancements in catalysis have paved the way for converting carbon dioxide into methanol, presenting a more sustainable route to fuel production using the kerosene molecule. These developments and strategies could potentially reshape the future of fuel production, steering it towards greater sustainability and cost-effectiveness.
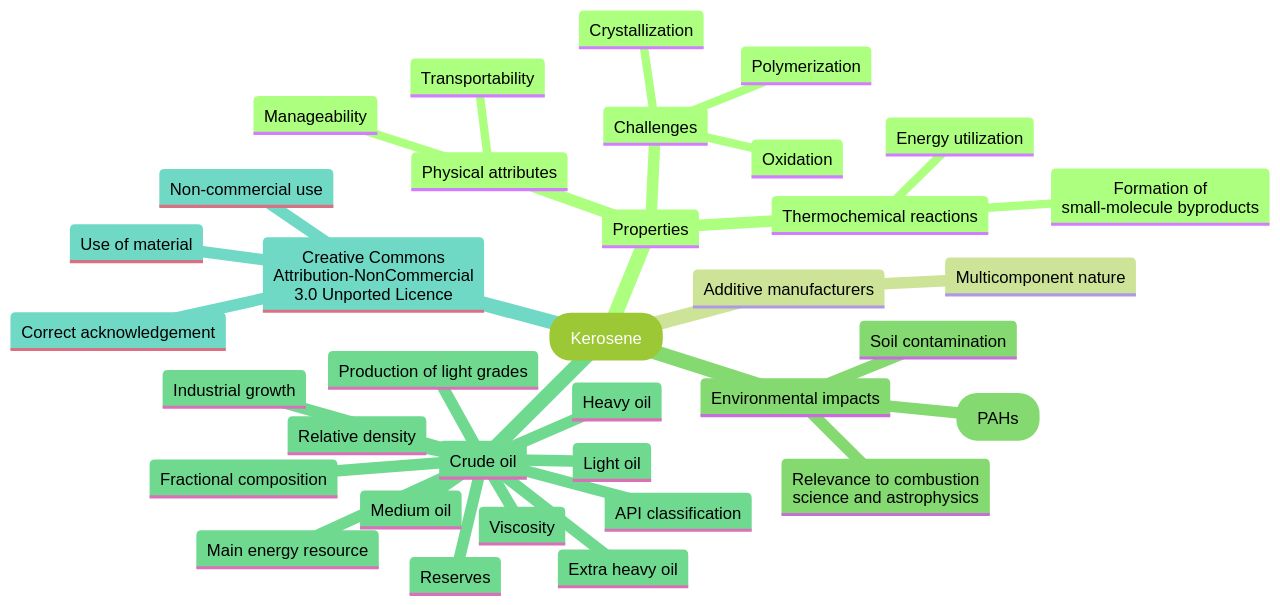
Physical and Chemical Properties
The kerosene molecule, being a transparent and low-viscosity liquid, possesses exceptional manageability and transportability due to its physical attributes. The kerosene molecule requires a higher ignition temperature compared to other fuels due to its high flashpoint, and its moderate boiling point allows for vaporization at temperatures that facilitate combustion.
In the energy utilization sector, thermochemical reactions of fuel are pivotal. Small-molecule byproducts from these reactions are more combustible, and the heat absorbed during cracking is released, thereby boosting the energy utilization rate.
However, the oxidation and polymerization reactions of the kerosene molecule in high-temperature conditions can lead to the formation of deposits within the narrow pipelines of aircraft and engine nozzles, posing a substantial safety risk. In addition, the technological challenge lies in the crystallization of n-alkanes in diesel fuels, particularly in cold climates, which is a hurdle for the kerosene molecule.
Additive manufacturers face a complex challenge due to the multicomponent nature of these fuels and its effect on crystallization, which involves the kerosene molecule. According to studies, soil contamination with the kerosene molecule can greatly alter the primary properties of the soil, nutrient provision for plants, and the supply of adequate air and heat for root systems. On a larger scope, the formation of polycyclic aromatic hydrocarbons (PAHs), which account for up to 30% of the carbon in the entire universe, is partly caused by the incomplete combustion of substances like coal and oil, involving the kerosene molecule. Recent research on the kerosene molecule has enhanced our understanding of the formation of PAHs, such as naphthalene, through gas-phase reactions. These insights into the properties and impacts of kerosene and related substances are vital for advancements in combustion science and astrophysics.
Role of Molecular Structure in Combustion
The complex process of fuel combustion is driven by the molecular structure of the kerosene molecule and gasoline. The energy of the kerosene molecule is obtained by breaking the carbon-carbon bonds in its long hydrocarbon chains during combustion.
The kerosene molecule's extremely low octane rating, around 30, makes it an unsuitable choice of fuel for gas engines as it can result in detonation and difficult ignition. On the contrary, the physical and chemical properties of gasoline, which is a blend of alkanes, naphthenes, olefins, and aromatic hydrocarbons, vary depending on the ratios of its components. These component ratios can be attributed to the presence of the kerosene molecule in the mixture.
This directly affects engine efficiency and emission of pollutants. The fuel undergoes thermochemical reactions, generating small-molecule products, including the kerosene molecule, that not only burn more readily but also release heat absorbed during the cracking reaction, enhancing energy utilization.
However, the presence of kerosene molecules in this process can lead to the formation of deposits in fine pipelines and engine nozzles, potentially posing a safety risk. Hence, it is crucial to understand the heat transfer deposition characteristics of fuels, especially the kerosene molecule in aviation and high-density endothermic hydrocarbon fuels. The deposition generated by oxidation reactions diffuses from the fuel to the surface of the reaction tube, while on the surface of the reaction tube, high-temperature pyrolysis reactions involving the kerosene molecule generate deposition in situ. The complexity of the reaction mechanism of gasoline, which is composed of various components including the kerosene molecule, increases exponentially, thus requiring a thorough comprehension of the combustion process of gasoline fuel. Raman spectroscopy quantitative analysis can be used to calculate the temperature distribution and composition distribution of the fuel in the reaction tube.

Implications for Different Applications
Kerosene's high energy content and low freezing point make it a crucial component in the aviation and heating sectors. However, the shift towards sustainable alternatives in the aviation industry is causing the role of kerosene to evolve.
For instance, the Netherlands is pioneering this shift by constructing factories to manufacture sustainable synthetic kerosene. Their goal is to transition European aviation to this fuel by 2050.
However, this transition to Sustainable Aviation Fuels (SAFs) is not without obstacles. The physical and combustion properties of aviation fuel necessitate the presence of aromatic molecules, despite their contribution to pollution.
While bio-based aromatics are being produced, the scale of production is insufficient, making Safe more expensive than traditional kerosene. This is where innovative solutions like Flexiforming technology by Unifuel.Tech come into play.
This technology, which can be implemented in an idle hydrotreater or reformer, offers a way to reduce both capital expenditure and carbon intensity. It provides operators with the flexibility to determine their pace of decarbonization. Unifuel.Tech requires details about the operator's feeds, target products, and existing facilities to find the optimal application for Flexiforming. Moreover, technologies like React™, which transform various feedstocks into low-carbon hydrogen or other chemicals and fuels, are aiding this transition. By pairing renewable electricity with natural gas, this technology eliminates flue gas emissions and becomes a significant contributor to the global shift to a low-carbon economy.

Conclusion
In conclusion, the analysis of the kerosene molecule provides valuable insights into its composition, properties, and implications across various applications. Advancements in synthetic kerosene production showcase its potential in a decarbonizing world.
Understanding polycyclic aromatic hydrocarbons (PAHs) sheds light on kerosene combustion and carbonaceous nanoparticle formation, benefiting combustion science and astrophysics. The chemical composition of kerosene presents opportunities for repurposing struggling refineries into renewable fuel production facilities.
Technologies like Flexiforming reduce capital expenditure and carbon intensity, allowing operators to determine their decarbonization pace. Physical and chemical properties of kerosene contribute to its manageability but pose challenges such as deposit formation.
Understanding these properties is vital for optimizing fuel utilization and ensuring safety. The molecular structure plays a significant role in combustion processes.
Clear understanding of fuel combustion processes is essential for improving engine performance. Implications include the shift towards sustainable alternatives like synthetic kerosene in aviation. Innovative technologies like Flexiforming reduce costs and carbon intensity in sustainable aviation fuel production. Pairing renewable electricity with natural gas through technologies like eREACT™ contributes to a low-carbon economy. Overall, this analysis highlights the complexity and potential of the kerosene molecule. By understanding its molecular structure and exploring innovative solutions, we can unlock opportunities for sustainable fuel production and contribute to a greener future.




